By Barry Keate
Barry Keate, has lived with tinnitus over 40 years and has published 150+ research articles on numerous aspects of tinnitus. He is an expert on the condition and a well-known advocate for those with tinnitus.
There’s been a lot of excitement in tinnitus circles recently surrounding the idea of repetitive Transcranial Magnetic Stimulation (rTMS) for the reduction of tinnitus symptoms. This innovative approach to tinnitus treatment now promises new hope for people plagued by constant tinnitus sounds.
A Brief History
For several decades, people with severe depression that was not responsive to medication have been treated as a last resort by Electro Convulsive Therapy (ECT), which we more normally refer to as Electro Shock Therapy. This technique has been shown to help severe depression which could be helped with no other treatment, however it is an extremely difficult process to withstand. ECT requires anesthetics and neuromuscular blocking medication to keep the patient still during the seizure induced by the procedure. Memory loss is a frequent side-effect.
rTMS was initially developed for mapping and measuring brain functionality. It is based on the principle that a varying magnetic field will cause an electrical current within any volume where it passes. Electromagnets with intense electric current are pulsed on and off immediately outside the skull. When the targeted rTMS magnetic field passes through the brain, it affects cortical neurons in a particular area. Early studies, beginning around 1985, found that the effect was not limited to the cortex but spreads out to the related subcortical structures. This finding gave researchers a basis for treating neural inactivity associated with some neuropsychiatric illnesses.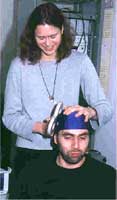
The typical apparatus for applying rTMS involves a capacitor which can deliver 5,000 to 8,000 amps of electric charge across a special magnetic coil. There are two types of coils: circular and double-circular, or figure eight. The double circular coils focus energy better and are generally used. Small coils deliver a higher intensity magnetic flux than larger coils but the intensity falls off quicker with distance. Therefore, small coils are typically used to treat superficial nerves while larger coils are used to treat deeper structures.
Early on in the research, grand mal seizures were induced in a small number of people by rTMS. The introduction of safety guidelines which limit the duration of treatment to 5 seconds or less have largely eliminated the undesirable seizures. It has also been established that no memory loss occurs as a result of the stimulation, even if seizures are intentionally induced.
Recent studies on rTMS vs. Electro Convulsive Therapy show that repetitive magnetic pulses of 10 Hz and 1Hz were both effective in relieving depression and mania. When compared to ECT, rTMS was found to be at least as effective and is much more humane than ECT. It can be conducted on an out-patient basis and patients generally report no side effects. Occasionally minor headaches or a general feeling of discomfort at the treatment site are reported. These effects usually resolve after a few treatments.
Expanding Therapeutic Benefits
During the past decade, researchers have explored and documented hundreds of beneficial uses for rTMS including schizophrenia, multiple sclerosis, post-traumatic stress disorder, Parkinson’s disease, obsessive-compulsive disorder and tinnitus. One finding was that low frequency rTMS reduced auditory hallucinations in patients with schizophrenia. A clinical trial is currently under way which will use rTMS to stimulate the brain of stroke patients in an effort to restore lost or damaged speaking ability.
Readers of this newsletter may recall the article we published in March, 2003 on electrical stimulation for the reduction of tinnitus. This article can be reviewed at HERE. This is an invasive therapy and, in the case of cochlear implants, can completely destroy hearing.
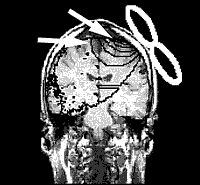
A team of researchers at the Department of Psychiatry and Psychotherapy, University of Regensburg, Germany have conducted a series of trials using rTMS on patients with tinnitus. Eleven patients underwent a PET (Positron Emission Tomography) scan to detect areas of increased metabolic activity in the cortex. This was coupled with a structural MRI scan to exactly identify the area of increased activity. A neuronavigational system was developed for rTMS to allow exact positioning of the figure eight shaped magnetic coil in relation to the target area.
This was a placebo controlled cross-over designed study. This means patients would undergo either the real rTMS or a placebo treatment, then cross over to the other treatment. Patients were blind regarding the tinnitus stimulation. A customized sham coil was used during the placebo phase.
In 10 of the 11 patients the researchers were able to localize an increased metabolic activity in the left superior temporal fold of the auditory cortex. After 5 days of rTMS treatment, a remarkable improvement of the tinnitus score was found using the standard tinnitus questionnaires. This effect could not be seen after the sham stimulation. These findings were presented at the American Academy of Otolaryngology-Head and Neck Surgery Foundation annual meeting in September, 2003.
It now appears that rTMS therapy shows great promise and may be the next development in electrical stimulation for tinnitus without the adverse side effects of an invasive therapy and destroyed hearing. There are many unanswered questions, such as; how safe is it? How long does the suppression last? Does it work for all kinds of tinnitus? These and other questions will be answered in future studies.
rTMS is already being routinely administered to patients in many countries throughout the world, with the notable exception of the US. The FDA has yet to approve it for ordinary use and requires oversight by local review boards, limiting usage to clinical trials and non-cortical stimulation.
2004 Arches Natural Products, Inc.
