Ginkgo Special Extract EGb 761 in the Treatment of Tinnitus
Holstein, N. Ginkgo Special Extract EGb 761 in the Treatment of Tinnitus. Fortschr. Med. 2001 Jan 11; 118(4) p. 157-164.
A survey of the results obtained in clinical trials
Summary
In the framework of a systematic literature survey, 19 clinical trials investigating the effects of tinnitus treatment with Ginkgo biloba special extract EGb 761 were identified and evaluated. The results of eight controlled studies on tinnitus due to cerebrovascular insufficiency or labyrinthine disorders of varying origin showed for the most part a statistically significant superiority of the treatment with Ginkgo biloba special extract EGb 761 as compared with placebo or reference drugs applied for periods of one to three months. Open studies, too, some involving large numbers of patients, revealed appreciable improvements under Ginkgo treatment, Therapeutic success was not directly correlated with either the genesis or the duration of tinnitus. However, investigations on prognostic factors showed that short-standing disorders have a better prognosis so that better results can be expected from early-onset treatment. The tolerability of Ginkgo biloba special extract EGb 761 was very good and, in this respect, the controlled clinical trials revealed little difference between active-substance groups and reference groups.
In the Western industrialized countries tinnitus is a rather common symptom. As related to the 0.1 – 0.2 % of the population suffering from acute functional disorders of the inner ear, 80-90% of them endure at the same time a transient or permanent ear noise. This affects in particular adults aged between 40 and 60 years – women more frequently than men. 35-45% of all adult Germans experience ear noises at least once in the course of their life, high-frequency noises being perceived more often than lower frequencies. In most cases, noises are described as whistling or roaring (38.8% and 27.9%), more seldom as squeaking, buzzing, hissing or ringing [15]
The varying forms of tinnitus do not only differ with respect to their sound and frequency but also as concerns loudness and periodicity. As compared to tinnitus which is only perceived subjectively, ear noises in case of objective tinnitus are assessable diagnostically, i.e. these noises can be perceived by external observers by means of a stethoscope. Independent of its intensity, tinnitus is so oppressing in l-2% of the patients that it leads to a decrease in working capacity and even to inability to work and suicide tendency (decompensated tinnitus). Depending on the duration of tinnitus, it is also classified in acute and chronic forms with chronic tinnitus meaning symptoms persisting for at least three months [15].
In about half the cases tinnitus is associated with an abnormal sensitivity to noise. Every fifth patient additionally complains about hardness of hearing [13]. Dizziness and cervical pain also frequently accompany tinnitus. Tinnitus is often induced by exceptional situations of life such as loss of one’s spouse or a close reference person, job or financial problems [22] which explains the importance of a good psychological assistance during therapy of a tinnitus patient. Clarification of possible somatic causes is primordial for trying to find an efficient way to help the tinnitus patient. Pulse-synchronic noises are indicative for a vascular process with arterial participation. Constant flow noises on the other hand occur in case of venous flow disorders or an increased vascular perfusion such as e.g. in anemia. Respiration-synchronic noises are heard in case of the so-called “gaping tube” and physiological activities of the intrinsic muscles of the middle ear (e.g. myoclonus of the musculus stapedius) or the tube muscles lead to clicking phenomena. Other diseases of the peripheral auditory system too, such as Meniere’s disease, sudden hearing loss, blast trauma or acute otitis media, may appear in combination with tinnitus. However, after exclusion of inflammatory and tumor-like causes, the etiology of acute functional disorders of the inner ear focuses on a vascular genesis [15].
The aim of a drug-based treatment in case of vascular causes is to improve microcirculation by reducing plasma viscosity and increasing the perfusion rate. Acute tinnitus treatment therefore starts with confinement to bed and rheological therapy. A further blood-flow promoting measure in acute tinnitus and in long-term treatment is the administration of Ginkgo special extract EGb 761. Depending on the cause and symptoms, calcium antagonists, antiarrhythmics, anticonvulsives and antidepressants are recommended, too [14].
The highly purified and concentrated monoextract EGb 761 obtained from the dried leaves of the Ginkgo biloba tree is a special extract manufactured according to a patented standardized pharmaceutical process. The combined effects of its components a.o. ginkgo flavone glycosides and terpene lactones (ginkgolides, bilobalide) results in a multifactor pharmacological action profile comprising a positive effect on rheological parameters and the energy metabolism of the nerve cells protecting them from the sequels of hypoxia and ischemia, and radical-scavenging properties [27]. This large action spectrum leads to its application in different fields of indication such as vertigo and tinnitus, peripheral arterial occlusive disease and dementia1 syndromes. The efficacy of the special extract EGb 761 is demonstrated for the above-mentioned indications by pharmacological and clinical studies [27]. The objective of the present systematic investigation is to give a survey on the efficacy and tolerability of Ginkgo special extract EGb 761 in the treatment of tinnitus aurium as based on the results of all clinical studies carried out with this extract in tinnitus patients.
Methods
All clinical trials documented in the scientific literature on the efficacy and tolerability of Ginkgo special extract EGb 761 in patients with subjective tinnitus were to be included in this survey. Restriction to studies with Ginkgo special extract EGb 761 is due to the problematic comparability of plant extracts from different manufacturers [16]. Data bases of EMBASE (unlimited until January 1998) and MEDLINE (unlimited until July 2000) were used for the identification of the studies. Results obtained with the search terms “ginkgo” and “tinnitus” showed that nearly all studies found were carried out with the Ginkgo special extract EGb 761. Upon request, the manufacturer of this extract made available further published studies which were not recorded in the data bases mentioned. From these identified clinical studies were taken into consideration all investigations in which Ginkgo special extract was administered orally for at least two weeks at a daily dose between 120 and 240 mg. We also included those studies in which tinnitus patients represented a sub-group of a patient population suffering from other inner ear disorders or disturbances of cerebral performance of vascular origin. For the selection of the studies, no restrictions were applied with respect to the study design, i.e. placebo and reference-controlled trials as well as open studies were included in this survey.
Results
After inspection of the published studies, 19 trials were selected on the base of the above-mentioned criteria, eight of which having been carried out under controlled conditions and eleven without reference groups. Subjective tinnitus symptoms were recorded in these studies according to an operational procedure defined in the trial plan. Clinical assessment was carried out via different rating scales measuring the subjective perception of ear noises and their changes under treatment. In addition, tone-audiometric measurements on tinnitus volume and other criteria were applied in some studies which, however, were used for assessing therapeutic success in only one case. Daily doses administered were between 120 and 240 mg and treatment periods reached from one month up to one year. In the framework of the literature search three other studies were selected which did not comply with the treatment criteria fixed since EGb 761 was either administered intravenously over merely eight days [25] or the therapeutic phase was preceded by a 10-day intravenous treatment with the Ginkgo special extract [21] or the oral daily dose was markedly below the search criteria with 2 x 14.6 mg [12]. These three studies were therefore not included into the survey of results.
Efficacy of Ginkgo special extract EGb 761 as compared with placebo or reference substances.
Table 1 shows the main data on patients, methods and results of the clinical trials carried out versus placebo or different reference substances.
Table 1: Controlled clinical trials with Ginkgo special extract EGb 761 as against placebo or reference substances in patients suffering from tinnitus.
|
Study
|
Patients
|
Methods*
|
Results
|
||||
| 1st author year [ ref.] |
Pat. nb. (with tinnitus)
|
Diagnosis
|
Special
data on tinnitus |
Study Design
|
EGb 761 daily d./ treatment duration
|
Refer. med./daily dose
|
Efficacy in tinnitus
|
|
Cheesebeouf
1979 [3] |
60 (29)
|
tinnitus, hearing deficit, dizziness
|
dif. origin, mostly
vascular |
mono-
center random. paral. groups, open |
3 x 40 mg/ 2 months
|
nicergolin
3 x 5 mg |
improve-
ment in both groups, no group diff. |
|
Eckmann
1982 [7] |
50 (32)
|
cerebrovasc. insuf-
ficiency a.o. with tinnitus |
cerebro-
vascular origin |
mono-
center, randomized, paral. groups double-blind |
3 x 40 mg/ 30 days
|
placebo
|
better efficacy under
Ginkgo biloba (p=0.04) |
|
Halama
1988 [11] |
40 (i.d.)
|
cerebrovasc. insuf-
ficiency a.o. with tinnitus |
cerebro-
vascular origin |
mon-
ocenter, random. paral. groups double-blind |
3 x 40 mg/ 12 weeks
|
placebo
|
improvement only under Ginkgo
biloba (p=0.035) |
|
Mangabeira
1986 [17] |
56 (i.d.)
|
tinnitus, balance discord. dizziness, hearing deficit
|
inner ear disorder
of different origin |
mono-
center, random. paral. groups double-blind |
3 x 40 mg/ 40 days
|
placebo
|
higher responder rate under Ginkgo
biloba |
|
Meyer
1986a [18] |
103 (103)
|
tinnitus & other sympt. (hearing deficit, dizziness)
|
duration
<1year |
multi-
center, random. paral. groups double-blind |
3 x 80 mg/ 3 months
|
placebo
|
better efficacy under
Ginkgo biloba (p=0.05) |
|
Meyer
1986b [19] |
259 (259)
|
tinnitus & other sympt. (hearing deficit, dizziness)
|
duration
<1year |
multi-
center, random. paral. groups open |
120 mg/
1 month |
nicergolin, almitri-
nraubasin, standard dose |
better efficacy under Ginkgo biloba (p<0.001)
|
|
Morgenstern
1997 [20] |
99 (99)
|
tinnitus
|
chronic
(>2 months) |
mono-
center run-in -phase random. paral. groups double -blind |
3 x 40 mg/ 12 weeks
|
placebo
|
better efficacy under
Ginkgo biloba (p=0.15) |
|
Natali
1979a [23] |
20 (13)
|
tinnitus, hearing deficit, dizziness
|
inner ear disorders
of vascular origin |
mono-
center, random. cross- over, open |
3 x 40 mg/ 2 x 1-2 months
|
cinnarizin,
3 x 20 mg |
mild to marked improve- |
| Total number 687 Patients (>535)*assessment criterion for tinnitus in all studies was rating scale; Morgenstern 1997 [20] additinally applied audiometric volume.i.d. = incomplete data on the number of tinnitus patients per treatment group |
|||||||
Early controlled studies compared the effect of EGb 761 with other active substances used for the treatment of disorders of the inner ear. In a cross-over study by Natali et al. 1979 [23] versus the vasodilator Cinnarizin, mild to marked improvements of ear noises were reported in 11 out of 13 patients treated with Ginkgo special extract as against mild improvements in two out of 13 patients in the Cinnarizin treatment phase. In a comparative study with the vasodilating substance Nicergolin [3], clear improvements without group differences were observed in 15 patients with ear noises of the Ginkgo special extract group and in 14 patients of the Nicergolin group as against the initial state.
Patients with cerebrovascular insufficiency or disorders of the inner ear of different origin also suffering partly from ear noises were investigated in three placebo-controlled clinical trials: In the study by Eckmann and Schlag 1982 [7] twelve patients treated with EGb 761 and 20 placebo patients suffered from tinnitus in the context of a cerebrovascular insufficiency. After a 30-day treatment, the ear noises were eliminated in all patients of the active-substance group whereas this symptom was still present in ten patients of the placebo group. This group difference was statistically significant (p = 0.04). Statistically remarkable differences in efficacy as compared to placebo and in favor of a treatment with Ginkgo biloba were also seen in a study by Mangabeira Albernaz et al. 1986 [17] involving non pre-treated patients with disorders of the inner ear of different origin. In total, improvement was achieved in 82.1% of the patients treated with EGb 761 (17 symptom-free, 6 improved) as against only 42.9% in the placebo group (three symptom-free, nine improved). As concerns the symptom tinnitus, an improvement was observed in five (31.3%) of the verum patients but in none of the placebo patients. In another study involving patients with mild to moderate cerebrovascular insufficiency too [11], a statistically significant decrease in ear noises could be demonstrated under EGb 761 whereas this symptom remained nearly unchanged under placebo (p = 0.035).
Exclusively tinnitus patients were examined in three controlled clinical trials with larger patient numbers:
103 patients aged as a mean 50 years and suffering from tinnitus and possibly concomitant symptoms such as dizziness and hypoacusis were involved in a multicenter double-blind study carried out by 10 E.N.T. specialists [18]. Patients for whom surgical, antibiotic or other medical treatments were indicated, were excluded from the study. After a twelve-week randomly allotted therapy with EGb 7618 or placebo, statistically significant group differences in favor of the patients treated with Ginkgo special extract were found. Tinnitus intensity decreased more markedly between the first and last date of examination under treatment with the active-substance than under placebo (p=0.03).
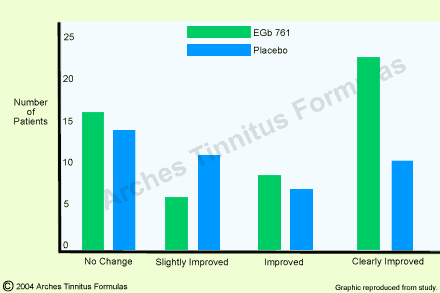
Global assessment of the therapeutic success too, showed a statistically significantly better result under Ginkgo biloba therapy (p = 0.05; fig. 1). Furthermore, treatment success was observed more quickly under EGb 761 than under placebo. Improvement or elimination of ear noises was achieved in 50% of the cases in the active-substance group as a mean after 70 days and in the placebo group only after 119 days (p=0.03). In addition, prognostic factors such as duration of disorder (longer or shorter than 30 days), localization (unilateral, bilateral) and periodicity (permanent, intermittent) were investigated in this study. Based on these factors, the patients were retrospectively distributed in five prognostic groups and analyzed with respect to the therapeutic success. It could be shown that Ginkgo special extract EGb 7618 lead to better therapeutic results in all prognostic groups and that globally, the factors “first occurrence”, “unilateral” and “intermittent” had a better prognostic value.
In an open multicenter study with similar design for the remaining traits [I9] and involving 259 patients in 50 E.N.T. practices, treatment with Ginkgo special extract was compared with the substances almitrin-raubasin and nicergolin also used for the treatment of disorders of the inner ear, administered at the usual therapeutic doses. With comparable starting conditions of the three therapeutic groups after randomization, the percentage of patients being symptom-free or improved was clearly higher in the Ginkgo special extract group with 63.3% as compared to nicergolin (38.8%) or almitrin-raubasin (29.7%). This group difference was statistically significant (p<0.00l).
Fig.1. Global assessment of tinnitus as compared to initial state after three-month
therapy in 103 patients with tinnitus (EGb 761 : n=55; placebo : n=45). Group difference was statistically significant with p = 0.05 [from 18].
In the framework of a placebo-controlled double-blind study carried out in a E.N.T. clinic [20], 99 outpatients suffering from chronic ear noises and with a mean duration of the disorder of 4.5 years were examined. The tinnitus whose sound type could be defined and which could be masked persisted for at least two months. Tinnitus intensity was measured audiometrically and by means of subjective assessment (rating scale). The audiometric measurement of the tinnitus volume showed a statistically significant group difference after twelve weeks of treatment (p=0.015; Fig.2). The improvements under Ginkgo special extract EGb 761 were situated within a range of 5 to 10 dB. As compared to these values, the values obtained in the placebo group remained nearly unchanged. For the subjective evaluation of the therapeutic success by the patients, 15 patients of the active-substance group (30.6%) as against only seven (14.0%) in the placebo group reported an improvement of their complaints at the end of thetreatment.
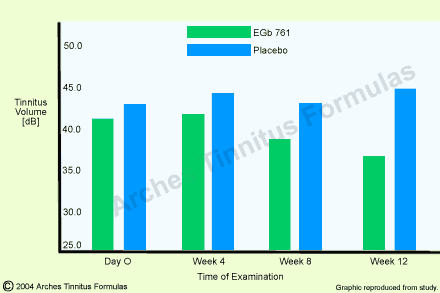
Fig.2: Tinnitus volume in dB on the ear more affected (mean value with 95% confidence intervals) in 99 patients with tinnitus (EGb 761 : n=49; placebo : n=50) and twelve-week treatment. Group difference was statistically significant with p=O.O15 [from 20].
Efficacy of Ginkgo special extract EGb 761 in clinical trials without reference groups.
Besides the controlled clinical trials listed, a great number of clinical trials without reference group were carried, too. These are for the one part smaller studies with pilot character but also important investigations on efficacy and tolerability in the daily practice. Table 2 gives a survey of the studies with the major data on the patients examined and the methods applied as well as on the results on efficacy in tinnitus treatment.
Table 2: Survey on clinical trials without reference group conducted with Ginkgo special extract EGb 761 in patients suffering from tinnitus.
|
Study
|
Patients
|
Methods*
|
Results
|
|||
| 1st author year [ ref.] |
Pat. nb. (with tinnitus)
|
Diagnosis
|
Special data on tinnitus
|
Study Design
|
EGb 761 daily d./ treatment duration
|
Efficacy in tinnitus
|
|
Artieres
1978 [1] |
42 (28)
|
diff. disorders of inner ear a.o. with tinnitus
|
varying intensity
|
open monocenter
|
120-160 mg/ 3 months
|
82% very good or good efficacy
|
|
CanoCuenca
19995 [2] |
70 (40)
|
diff. disorders of inner ear a.o. with tinnitus
|
vascular orign
|
open multicenter
|
2 x 80 mg/ 6 months
|
improvements during treatment (p=0.0001)
|
|
Claussen
1981 [4] |
50 (50)
|
dizziness and tinnitus
|
varying origin and duration
|
open monocenter
|
3x 80 mg/ (1st month) 3x 40 mg (2nd month)
|
improvement in 1/3 of the cases
|
|
Coles
1988 [5] |
23 (23)
|
tinnitus
|
duration>3 years in 18 patients (median 8.5 years)
|
open, pilot study
|
3 x 40 mg/ 12 weeks
|
no efficacy
|
|
D’Avila
1992 [6] |
885 (566)
|
cerebral and peripheral circulatory disturbances
|
symptom in the context of basic disease
|
open multicenter
|
3 x 40 mg/ 60 days
|
continuous regression during treatment
|
|
Gananca
1986 [9] |
360 (68)
|
diff. disorders of inner ear a.o. with tinnitus
|
varying origin pre-treated without success
|
open monocenter
|
3 x 40 mg/ 90 days
|
improvement in 18 of 68 patients
|
|
Gomez
1997 [10] |
202 (20)
|
memory disorders, dizziness, tinnitus
|
open multicenter
|
3 x 40 mg/ 12 weeks
|
improvement in most patients
|
|
|
Natali
1979b [24] |
68 (44)
|
tinnitus, hearing deficit, dizziness
|
varying intensity
|
open monocenter
|
3 x 40 mg/ average 4 months (11 pat.>12 months)
|
freedom from symptoms in 43%, improvement in 29.5%
|
|
Pietra Santa
1990 [26] |
1368 (862)
|
cerebrovascular insufficiency
|
symptom in the context of basic disease
|
open multicenter
|
3 x 40 mg/ 40 days
|
82% free of symptoms at end of treatment (p<0.001)
|
|
Sprenger
1986 [28] |
64 (33)
|
cochlear deafness, partly retrospective, with tinnitus
|
open monocenter
|
3 x 40 mg/ 9 weeks
|
freedom from symptoms in 36%, improvement in 15% of the patients
|
|
|
Vorberg
1985 [29] |
112 (68)
|
chronic cerebrovascular insufficiency
|
symptom in t he context of basic disease
|
open multicenter
|
3 x 40 mg/ 1year
|
continuous decrease of tinnitus, before-after comp. (p<0.001)
|
| Total number 3244 Patients (1802)*assessment criterion for tinnitus in all studies was rating scales; |
||||||
The 1802 tinnitus patients involved suffered from ear noises in the context of cerebra-vascular circulation disorders or from disorders of the inner ear of different origin with symptom complexes including ear noises relatively frequently. With different treatment periods between 40 days and six months, more or less pronounced improvements of the tinnitus symptoms or elimination of the ear noises were obtained in most studies.
Safety and tolerability of Ginkgo special extract EGb 761.
In all clinical trials, safety and tolerability of the medication with Ginkgo special extract were very good and good. In as far as mild disorders or treatment discontinuations occurred due to side-effects, no pronounced differences between the EGb 761-treated groups and the respective reference groups were found in the controlled clinical studies. From uncontrolled investigations conducted with large number of patients, too, no serious or unexpected cases of suspected adverse drug reactions could be deduced from the results obtained. The adverse effects for which the physician or the patient assumed a relationship with the medication, were mild disorders such as gastro-intestinal symptoms, headache or tiredness. In those cases in which laboratory parameters were measured during the study, no medication-related changes were observed.
Discussion
The results of this systematic literature survey show that the effect of Ginkgo special extract EGb 761 in tinnitus patients was investigated in numerous studies partly involving large patient numbers. The superior efficacy of EGb 761 as compared to reference groups could be verified statistically in the five placebo-controlled studies. In one of these studies, the audiometrically measured decrease in tinnitus volume was within a range of 5 to 10 dB which is to be considered as clinically relevant [20]. The three reference-controlled studies showed that the effects of Ginkgo special extract EGb 7618 can be rated as comparable [3] to significantly better [19, 23] as against the vasodilators nicergolin, almitrin-raubasin and cinnarizin. Therapeutic periods after which clear symptom improvements could first be seen, were between one and three months. In nearly all uncontrolled studies too, clinical improvements of tinnitus symptoms were found in partly very large numbers of patients. Since ear noises frequently persisted for a longer time already and the spontaneous healing rate could thus be expected to be rather low, the results of the uncontrolled clinical studies are also important for the proof of efficacy. Tolerability of the Ginkgo special extract EGb 761 was very good to good in all investigations. An essential difference between EGb 761 groups and the respective reference groups could not be shown.
As concerns the effect of EGb 761, no direct connection with specific prognostic factors could be detected in the investigations. This applies in particular to the genesis of the tinnitus symptoms. Patients with tinnitus of varying origin were examined in the clinical studies performed. For the majority of the cases, the most probable cause was a cerebrovascular insufficiency, but the studies also investigated a large number of patients with disorders of the inner ear of different or not clarified origin and with variable symptoms. The results did not indicate a relation between the efficacy of Ginkgo special extract EGb 761 and the tinnitus genesis. The investigations showed a clinical efficacy of EGb 761 in case of acute and chronic tinnitus auris. Two large-scale studies, however, established that in case of shorter durations of the disease a better prognosis and treatment success are to be expected in general [18, 24]. The clinically relevant conclusion to be drawn from this, is to start treatment as early as possible.
As based on the present results from clinical studies, it can be stated as summary that Ginkgo special extract EGb 761 is an effective and very well tolerated medication which can be applied for the treatment of ear noises of varying origin and duration. Pharmaco-economic aspects are also to be taken into consideration for the practical importance of such a treatment. In fact, it is often not possible on the one hand to find a tinnitus origin that can be treated causally, but on the other hand, the ear noises are subjectively very stressing for the patient. This often leads to frequent medical consultations, ineffective treatment attempts and incapacity to work which produces considerable costs that could be prevented by an early effective therapy. The present systematic literature review thus also contributes to the conception of evidence based medicine in the treatment of tinnitus. The authors of a recently published survey on the treatment of tinnitus patients with Ginkgo extracts [8] in which, however, merely four controlled clinical studies with the Ginkgo special extract EGb 761 [12, 18-20] and one study with a Ginkgo extract of another manufacturer were evaluated, also come to the conclusion that the results are indicative for a therapeutic benefit of Ginkgo extracts in tinnitus treatment. Further controlled investigations in accordance with present methodic requirements would be desirable for corroborating these results.
Conclusion
The results of a systematic survey on the treatment of tinnitus patients with EGb 761 in the framework of clinical studies show the efficacy and good tolerability of Ginkgo special extract as compared to placebo or reference substances. The efficacy could be demonstrated in tinnitus of different origin and duration, but prognosis is generally better in case of short duration of the disease so that treatment should be started as early as possible.
I would like to thank the company Dr. Willmar Schwabe Arzneimiffel, Karlsruhe (Germany) for the complementary literature provided.
References
1. Artieres, J.: Effets therapeutiques du tanakan sur les hypoacousies et les acouphenes. Lyon Med. 14(1978),2503-2515.
2. Cano Cuenca, B., Marco Algarra, J., Perez del Valle, B., Pellicer Pascual, F.J.: Efecto del Ginkgo biloba sobre la patologia cocleovestibular de origen vascular. An. Otorinolaringol. Iber.-Amer.22(1995), 619-629.
3. Chesseboeuf, L., Herard, J., Trevin, J.: Etude comparative de deux vasoregulateurs dans les hypoacousies et les syndromes vertigineux. Med. Nord Est 3(1979),534-539.
4. Claussen, E., Claussen, C.-F.: Eine Vergleichsstudie zur Behandlung von Schwindel und Tinnitus mit Rokan.In: Claussen, C.-F.: Verhandlungen der Gesellschaft fur Neurootologie und Aequilibriometrie e. V., Gleichsgewichtsprufungen und Arbeitsmedizin. Band VIII. Verlag edition m+p, Hamburg und Neu-Isenburg 1981, S. 471-485.
5. Coles, R.: Trial of an extract of Ginkgo biloba (EGB) for tinnitus and hearing loss. Clin. Otolaryngol. 13(1998),501-502.
6. D’Avila, J.L.S.: Estudo multicentrico da eficacia do extrato de Ginkgo biloba (EGb 761) em pacientes com unsufficiencia vascular cerebral e periferica. Arq. Brasill. Med. 66(1992),87-91.
7. Eckmann, F., Schlag, H.: Kontrollierte Doppelblind-Studie zum Wirksamkeitsnachweis von Tebonin forte bei Patienten mit zerebrovaskularer Insuffizienz. Fortschr. Med. 100(1982),1474-1478.
8. Ernst, D., Stevinson, C.: Ginkgo biloba for tinnitus: a review. Clin. Otolaryngol. 24(1999),164-167.
9. Gananca, M.M., Mangabeira Albernaz, P.L., Caovilla, H.H., Ito, Y.I.:Ginkgo biloba no tratamento da vertigem e outros sintomas labirinticos. A Folha Medica 93(1986).
10. Gomez, A.E.: Estudio multicentrico del extracto estandarizado de Ginkgo biloba en el tratamiento de los trastornos de la memoria, vertigo Y tinnitus. Invest. Med. Int. 24(1997),31-39.
11. Halama, P., Bartsch, G., Meng, G.: Hirnleistungsstorungen vaskularer Genese. Fortschr. Med. 106(1988),408-412.
12. Holgers, K.-M., Axelsson, A., Pringle, I.: Ginkgo biloba extract for the treatment of tinnitus. Audiology 33(1994),85-92.
13. Kellerhals, R., Zogg, R.: Tinnitus. Ther. Umschau 52(1995), 718-723.
14. Lenarz, T.: Ohrgerausche: Ursachen, Diagnostik und Therapiemoglichkeiten. Med. Monatsschr. Pharm. 14(1991), 292-300.
15. Lenarz, T.: Allgemeine Diagnostik und Differentialdiagnose. In: Feldman, H.: Tinnitus. Georg Thieme, Stuttgart und New York 1992. S. 76-83.
16. Loew, D., Habs, M., Klimm, H.-D., Trunzler, G.: Phytopharmaka-Report: Rationale Therapie mit pflanzlichen Arzneimittein. 2. Auflage. Steinkopff, Darmstadt 1999.
17. Mangabeira Albernaz, P.L., Malavasi Gananca, M., Ito, Y.I., Caovilla, H.H.: Estudo duplo-cego da atividade do extracto de Ginkgo biloba no tratamento das labirintopatias. A Folha Medica 93,(1986),375-377.
18. Meyer, B.: Etude multicentrique randomisee a double insu face au placebo du traitement des acouphenes par l’extrait de Gingko biloba. Presse Med. 15(1986a),1562-1564.
19. Meyer, B.: Etude multicentrique des acouphenes. Epidemiologie et therapeutique. Ann. Oto-Laryng. 103(1986b),185-188.
20. Morgenstern, C., Biermann, E.: Ginkgo Spezialextrakt EGb 761 in der Behandlung des Tinnitus aurium. Ergebnisse einer randomisierten, doppelblinden, plazebokontrolleirten Studie. Fortschr. Med. 115, Originalien IV(1997),7-11.
21. Morgenstern, C., Biermann, E.: Die Wirksamkeit von Ginkgo-Spezialextrakt EGb 761 bei Patienten mit Tinnitus. Publikation in Vorbereitung.
22. MRC Institute of Hearing Research: Epidemiology of tinnitus. In: Hazell, J.W.P.: Tinnitus. Churchill Livingstone, Edinburgh 1987, S. 46-70.
23. Natali, R., Rachinel, J., Pouyat, P.-M.: Essai comparatif croise en O.R.L. de deux medications vasoactives. Cah. D’O.R.L. 14(1979a),185-190.
24. Natali, R., Rachinel, J., Pouyat, P.-M.: Le Tanakan dans les syndromes cochleovestibulaires relevant d’une etiologie vasculaire. Traitement de long cours. Gaz. Med. Fr. 86(1979b),1381-1384.
25. Olivier, J., Plath, P.: Combined low power laser therapy and extracts of Ginkgo biloba in a blind trial of treatment for tinnitus. Laser Ther. 5(1993),137-139.
26. Pietra Santa, C.E., Garcia Aguilar, M.A.: Efficacia del extracto estandarizado de Ginkgo-Biloba EGB 761 en el tratamiento de las insufficiencia vascular cerebral. Invest. Med. Int. 17(1990),130-141.
27. DeFeudis, F.V.: Ginkgo biloba extract (EGb 761). From chemistry to the clinic. Ullstein Medical Verlagsgesellschaft, Weisbaden 1998.
28. Sprenger, F.-H.: Gute Therapieergebnisse mit Ginkgo biloba. Arztl. Praxis 29(1986),938-940.
29. Vorberg, G.: Ginkgo biloba extract (GBE): A long-term study of chronic cerebral unsufficiency in geriatric patients. Clin. Trials J. 22(1985),149-157.